- About Us
- Clinical Development
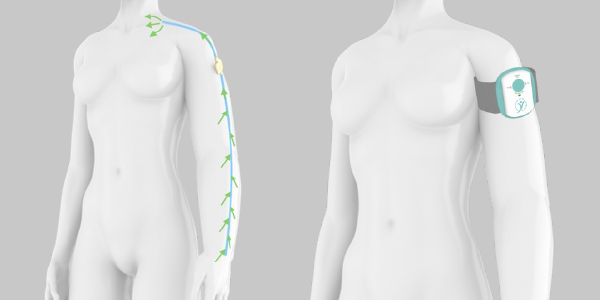
Lymphatica Medtech is a medical device company founded to develop LymphoDrain, the first artificial lymphatic bypass for lymphedema treatment. LymphoDrain™ is a minimally invasive implant controlled by an external wearable device, designed to re-establish lymphatic circulation in a discrete and seamless way. Through a connected application for patients and doctors, LymphoDrain™ will allow treatment personalization and monitoring of device function.
Watch how Lymphodrain™ technology works.
To know more about LymphoDrain™ technology, click here.

If you wish to be part of our story and invest in Lymphatica, click here.
LymphoDrain™ is currently under clinical investigation and it is not available on the market. The first version of LymphoDrain™ has been designed to treat arm lymphedema post breast cancer, and it is currently being used in the LymphoPilot clinical study at the University Hospital of Lausanne in Switzerland (CHUV). Find out more.
The LymphoPilot clinical study is a first-in-man study to assess feasibility and safety of the investigational implantable device LymphoPilot, and to collect preliminary data on lymphedema outcome measures. LymphoPilot is composed of an implanted part and an external wearable device. The implanted part (pump, drainage catheter and output catheter), is implanted in the subcutaneous tissue of the arm and drains excess fluids to the supraclavicular subcutaneous tissue, where fluids are naturally reabsorbed by the healthy lymphatic and venous system. The external part consists in a wearable controller used to activate the implanted pump.
If you are interested in participating in the LymphoPilot study, please click here to find the investigational site contact information.