A guest blog post by Melissa B. Aldrich, M.B.A., Ph.D. This is part one of a two-part series about Dr. Aldrich and her colleagues’ clinical imaging studies and emerging research about the development of lymphedema. It is a 7-minute read.
As a Ph.D. immunologist, I am part of a team that includes biomedical engineers, biologists, and chemists, all working together on pre-clinical and clinical imaging studies with collaborating physicians and therapists. Currently, I and my colleagues and I are running a longitudinal imaging study of breast cancer-related lymphedema (BCRL), which is an incurable condition many women face after going through breast cancer treatment. Another study investigating lymphatic microsurgeries has just begun.
An important part of any lymphatic imaging toolbox is near-infrared fluorescence lymphatic imaging (NIRF-LI ) [1], which allows cross-validation of animal and human studies. NIRF-LI lets you “see through your skin” so that you can tell if lymph is pumping normally. Other clinical and investigational lymphatic imaging tools include lymphoscintigraphy, magnetic resonance lymphography (MRL), single photon emission computerized tomography (SPECT-CT), and photoacoustic tomography [2].
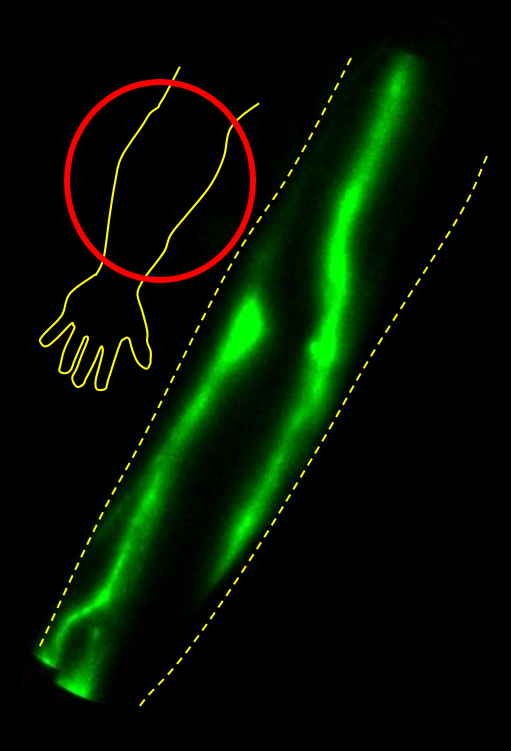 The NIRF-LI image above shows lymphatic vessels in an arm. NIRF-LI also provides movies of the lymph pumping.
The NIRF-LI image above shows lymphatic vessels in an arm. NIRF-LI also provides movies of the lymph pumping.
NIRF-LI, developed by my colleagues, is allowing investigation into how the lymphatics operate in many contexts. NIRF-LI has enabled animal studies of cancer, rheumatoid arthritis, and inflammation, baby chylothorax studies, and human studies of lipedema, primary lymphedema, secondary lymphedema, pneumatic compression therapy, rare adipose disorders, and combined lymphatic and vascular anomalies [3-11].
As the title of this article suggests, these studies will hopefully lead to a greater understanding of why certain people seem to be prone to developing lymphedema. It’s perplexing that, in some people, a switch flips and leads to lymphedema, but in others, that switch never flips.
Latent Lymphedema and Potential Triggers
Here’s something important we’ve learned so far: some people seem to have what might be called latent lymphedema. Many people who develop cancer-related lymphedema don’t experience swelling until a very long time after their cancer treatment ends. They can live for years post-cancer treatment before obvious lymphedema (swelling) emerges and begins to impact their health. Yet, in many of our study subjects, NIRF-LI shows that lymphatic flow is disrupted or abnormal well before any swelling appears.
This is perhaps the most convincing time-bomb evidence we have so far. Something is triggering the emergence of obvious lymphedema with swelling - and it’s usually not a gradual occurrence but a sudden onset. Why is this happening?
This leads to several other compelling questions:
- Do many people carry genes that predispose to lymphedema after cancer treatment or a traumatic injury [12]?
- What are the “second hits” that push latent lymphedema into obvious swelling?
Re-evaluating the Basic Facts About Lymphedema
We are also taking a fresh look at how many people truly have lymphedema. Estimates show there are about 50,000 primary lymphedema cases in the U.S., and that 40% of those with primary lymphedema have mutations that are known, while the other 60% have unknown mutations. But are these estimates accurate [13,14]?
For example, look at the Turner Syndrome population. Almost all of these patients are born with lymphedema - puffy hands and feet - which seems to go away during childhood, then return in adulthood for some patients. A lymphoscintigraphy study found that at least 80% of them actually have dysfunctional lymphatics, even though only 2/3 report lymphedema symptoms anytime during life [15, 16].
What’s happening here? Is this phenomenon attributable to the fact that these patients may have engaged in more physical activity during childhood? Or could it be that mild lymphedema is easily overlooked in Turner’s Syndrome, just as it could be in someone who is a cancer survivor? Or even someone within the general population?
This is why my colleagues and I are working to shed light on these issues through the use of imaging studies. We’ve found that, in breast cancer patients, many lymphatic vessel anatomical and pumping abnormalities occur well in advance of clinical lymphedema, when swelling and skin changes become evident.
There is also compelling evidence that chemotherapy contributes to lymphedema development. Research by other groups shows that doxorubicin, a cancer treatment drug, can disrupt lymphatic pumping [17]. Perhaps, in some people, chemotherapy serves as the “first hit” that begins to trigger lymphedema, and if they receive another “hit,” lymphedema fully develops.
More Unanswered Questions About Lymphedema
It’s interesting to compare and contrast lymphedema with lipedema, a genetic condition that does not set in until early puberty and appears to be estrogen-related [18, 19]. Lipedema and lymphedema are separate conditions, but in some patients with lipedema, lymphedema develops at late stages.
Lipedema has us wondering what kinds of “second hits” are turned on by puberty hormones. What is disrupting lymph pumping in the lipedema population? Why does this affect a whopping 11% of women? Is it an evolutionary advantage of some kind? What does this imply in terms of lymphedema? More research will provide answers to these questions.
There’s also the relationship between having a high BMI and developing lymphedema [20]. Higher BMI is usually considered a risk factor for developing secondary lymphedema, yet our NIRF-LI studies clearly show that slim breast cancer patients, with BMIs of 20-22, can develop lymphatic dysfunction, with no apparent arm swelling. Is it possible that these women have latent lymphedema?
NIRF-LI has provided so many insights into lymphedema, yet there are still so many unanswered questions. These questions are at the heart of our research, and they fuel my personal passion for this field of study.
In the next part of our two-part series, Dr. Aldrich will review the impact of exercise on lymphatic function and describe how imaging is helping with the early detection of lymphedema.
About Dr. Aldrich
Melissa Aldrich, M.B.A., Ph. D is an immunologist who works as part of a team of biomedical engineers, biologists, and other professionals who are investigating lymphatics in a variety of contexts. Dr. Aldrich received her B.A. in Biochemistry from the University of Texas-Austin, her M.B.A. from the University of Houston, and Ph.D. in Immunology from the University of Texas Health Science Center/M.D. Anderson Cancer Center Graduate School of Biomedical Sciences in Houston, focusing on severe combined immune deficiency. Currently, Dr. Aldrich is leading a five-year NIH-funded study of breast cancer patients who are at high risk of immunological changes and the development of lymphedema, and a new three-year study of lymphatic microsurgeries. Click here to see more about her background and credentials.
References
Rasmussen, J.C.; Tan, I.C.; Marshall, M.V.; Adams, K.E.; Kwon, S.; Fife, C.E.; Maus, E.A.; Smith, L.A.; Covington, K.R.; Sevick-Muraca, E.M. Human lymphatic architecture and dynamic transport imaged using near-infrared fluorescence. Transl Oncol 2010 3, 362-372
Munn, L.L.; Padera, T.P. Imaging the lymphatic system. Microvasc Res 2014 96, 55-63.
Kwon, S.; Velasquez, F.C.; Sevick-Muraca, E.M. Near-infrared fluorescence lymphatic imaging in vascular endothelial growth factor-C overexpressing murine melanoma. Biomed Opt Express 2018 9, 4631-4637. DOI: 10.1364/BOE.9.004631
Aldrich, M.B.; Velasquez, F.C.; Kwon, S.; Azhdarinia, A.; Pinkston, K.; Harvey, B.R.; Chan, W.; Rasmussen, J.C.; Ross, R.F.; Fife, C.E.; Sevick-Muraca, E.M. Lymphatic delivery of etanercept via nanotopography improves response to collagen-induced arthritis. Arthritis Res Ther 2017 19, 116.
Aldrich, M.B.; Sevick-Muraca, E.M. Cytokines are systemic effectors of lymphatic function in inflammation. Cytokine 2013, 64, 362-9.
Gonzalez-Garay, M.L.; Aldrich, M.B.; Rasmussen, J.C.; Guilliod, R.; Lapinski, P.E.; King, P.D.; Sevick-Muraca, E.M. A novel mutation in CELSR1 is associated with hereditary lymphedema. Vascular Cell 2016 8, 1.
Rasmussen, J.C.; Tan, I-C.; Naqvi, S.; Aldrich, M.B.; Maus, E.A.; Blanco A.I.; Karni, R.J.; Sevick-Muraca, E.M. Longitudinal monitoring of the head and neck lymphatics in response to surgery and radiation. Head Neck 2017 39, 1177-1188. PMID: 28263428
Aldrich, M.B.; Gross, D.; Morrow J.R.; Fife, C.E.; Rasmussen J.C. Effect of pneumatic compression therapy on lymph movement in lymphedema-affected extremities, as assessed by near-infrared fluorescence lymphatic imaging. J Innov Opt Health Sci 2017 10, 1650049.
Rasmussen, J.C.; Aldrich, M.B.; Tan, I-C.; Darne, C.; Zhu, B.; O’Donnell Jr., T.F.; Fife, C.E.; Sevick-Muraca, E.M. Lymphatic transport in patients with chronic venous insufficiency and venous leg ulcers following sequential pneumatic compression. J Vasc Surg Venous 2016 4, 9-17.
Rasmussen, J.C. Aldrich, M.B., Guilliod R., Fife, C.E., O’Donnell Jr., T.F.; Sevick-Muraca, E.M. near-infrared fluorescence lymphatic imaging in a patient treated for venous occlusion. J Vasc Surg Cases 2015 1, 201-204.
Rasmussen, J.C.; Herbst, K.L.; Aldrich, M.B.; Carne, C.D.; Tan, I-C.; Zhu, B.; Guilliod, R.; Fife, C.E.; Maus, E.A.; Sevick-Muraca, E.M. An abnormal lymphatic phenotype is associated with subcutaneous adipose tissue deposits in Dercum’s disease. Obesity 2014 22, 2186-92.
Miaskowski, C.; Dodd, M.; Paul, S.M.; West, C.; Hamolsky, D.; Abrams, G.; Cooper, B.A.; Elboim, C.; Neuhaus, J.; Schmidt, B.L.; Smoot, B.; Aouizerat, B.E. Lymphatic and angiogenic candidate genes predict the development of secondary lymphedema following breast cancer surgery. PLoS One 2013 8, e60164.
lymphnotes.com/article/php/id/307/
Mendola, A.; Schlogel, M.J.; Ghalamkarpour, A.; Nguyen, H.L.; Fastre, E.; Bygum, A.; van der Bleuten, C.; Fagerberg, C.; Baselga, E.; Quere, I.; Mulliken, J.B.; Boon, L.M.; Brouillard, P.; Vikkula, M.; Lymphedema Research Group. Mutations in the VEGFR3 signaling pathway explain 36% of familial lymphedema. Mol Syndromol 2013 4, 257-66.
Gellini, C.; Di Battista, E.; Boccardo, F.; Campisi, C.; Villa, G.; Taddei, G.; Traggiai, C.; Amisano, A.; Polo Perucchin, P.; Benfenati, C.S.; Bonioli, E.; Lorini, R. The role of lymphoscintigraphy in the diagnosis of lymphedema in Turner syndrome. Lymphology 2009 42, 123-9.
Welsh, J.; Todd, M. Incidence and characteristics of lymphedema in Turner’s syndrome. Lymphology 2006 39, 152-153.
Stolarz, A.J.; Sarimollaoglu, M.; Marecki, J.C.; Fletcher, T.W.; Galanzha, E.I.; Rhee, S.W.; Zharov, V.P.; Klimberg, V.S.; Rusch, N.J. Doxorubicin activates ryanodine receptors in rat lymphatic muscle cells to attenuate rhythmic contractions and lymph flow. J Pharmacol Exp Ther 2019 371, 278-289. doi: 10.1124/jpet.119.257592. PMID: 31439806.
Wiedner, M.; Aghazanzadeh, D.; Richter, D.F. Differential diagnoses and treatment of lipedema. Plast Aesthet Res 2020 7, 10.
Morfoisse, F., Tatin, F.; Chaput, B.; Therville, N.; Vaysse, C.; Metivier, R.; Malloizel-Delaunay, J.; Pujol, F.; Godet, A.C.; De Toni, F.; Boudou, F.; Grenier, K.; Dubuc, D.; Lacazette, E.; Prats, A.C.; Guillermet-Buibert, J.; Lenfant, F.; Garmy-Susini, B. Lymphatic vasculature requires estrogen receptor-alpha signaling to protect from lymphedema. Arterioscler Thromb Vasc Biol 2018 38, 1346-1357.
Sayegh, H.E.; Asdourian, M.S.; Swaroop, M.N.; Brunelle, C.L.; Skolny, M.N.; Salama, L.; Taghian, A.G. Diagnostic methods, risk factors, prevention, and management of breast cancer-related lymphedema: past, present, and future directions. Curr Breast Cancer Rep 2017 9, 111-121.
